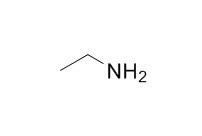
Ethylamine
Ethylamine and glutamic acid are substrates of theanine synthetase.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Sci.2019, 20(8):E1855
Biochem Biophys Res Commun.2021, 534:802-807.
Korea Institute of Oriental Medicine2020, doi: 10.21203.
Foods.2021, 10(12):2929.
Journal of Functional Foods2022, 99: 105331.
Industrial Food Engineering2015, 19(4):408-413
Evid Based Complement Alternat Med.2022, 2022:3483511
South African J of Plant&Soil2018, 29-32
Journal of Natural Remedies2024, 24(3):555ĘC575.
Int J Mol Sci.2023, 25(1):283.
Related and Featured Products
Z Naturforsch C. 2009 May-Jun;64(5-6):387-90.
Ethylamine content and theanine biosynthesis in different organs of Camellia sinensis seedlings[Pubmed:
19678543]
METHODS AND RESULTS:
We examined the distribution of Ethylamine, glutamic acid and alanine, which are utilized in theanine biosynthesis, and other major amino acids in leaves, stems, cotyledons and roots of 6-week-old tea seedlings. Ethylamine and glutamic acid, which are substrates of theanine synthetase, were distributed almost uniformly in all parts of the seedlings; the contents in micromol/g fresh wt varied from 0.44-0.88 (Ethylamine) and 1.6-2.4 (glutamic acid). The content of alanine, a possible precursor of Ethylamine synthesis, was significantly higher in roots (3.1 micromol/g fresh wt) than in other parts. Incorporation of radioactivity from [U-14C]-alanine into theanine was also higher in roots than in other organs. In 10-week-old seedlings, [1-14C]Ethylamine was converted to theanine in young and developed leaves, stems, main and lateral roots; the highest rates of conversion were detected in the main and lateral roots.
CONCLUSIONS:
These results suggest that the theanine synthesis preferentially takes place in roots but is not restricted to them; substrates and the enzymatic machinery for theanine synthesis are available in all parts of tea seedlings.
J Colloid Interface Sci. 2014 Aug 15;428:295-301.
Efficient removal of cesium from aqueous solution with vermiculite of enhanced adsorption property through surface modification by ethylamine.[Pubmed:
24910065]
Ethylamine modified vermiculite (Ethyl-VER) with high specific surface area and excellent pore structure was prepared to remove cesium from aqueous solution.
METHODS AND RESULTS:
The physic-chemical properties of the pristine and modified vermiculite were analyzed by X-ray diffraction (XRD), Fourier-transform infrared (FTIR), specific surface area (BET) and scanning electron microscopy/energy disperse spectroscopy (SEM/EDS). The corroding effect of Ethylamine increased the specific surface area of vermiculite from 4.35 to 15.59 m(2) g(-1), and the average pore diameter decreased from 6.8 to 5.34 nm. Batch adsorption experiments were conducted as a function of pH, initial Cs(+) concentration, contact time, coexisting cations (K(+), Na(+), Ca(2+)) and low-molecular-weight organic acids (acetic acid, oxalic acid, citric acid) to illustrate the adsorption behavior. The study found that the adsorption capacity of cesium in aqueous solution was improved from 56.92 to 78.17 mg g(-1) after modification. The formation of micropores and mesopores and the increased surface area played a critical role in the enhancement of cesium adsorption. Kinetic experiments indicated that the adsorption process can be simulated well with a pseudo-second-order model. The presence of cations or low-molecular-weight organic acids inhibited cesium adsorption in different degrees.
CONCLUSIONS:
On the basis of our results, Ethyl-VER with good surface characteristics and high adsorption capacity is a suitable adsorbent for cesium removal from aqueous solution.