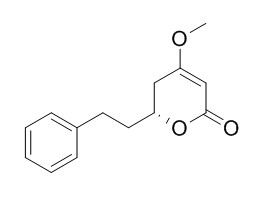
Dihydrokavain
Dihydrokavain may play an important role in regulation of GABAergic neurotransmission, it non-competitively inhibits the specific binding of [3H]-batrachotoxinin-A 20-alpha-benzoate to receptor site 2 of voltage-gated Na+ channels. Dihydrokavain may treat sleep disturbances, as well as stress and anxiety.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Applied Biological Chemistry2022, 65(77).
Antibiotics (Basel).2024, 14(1):8.
Tumour Biol.2015, 36(9):7027-34
Pharmacol Rep.2020, 72(2):472-480.
Toxicological Research2020, doi: 10.1007.
BMC Complement Altern Med.2014, 14:352
J Nutr Biochem.2022, 107:109064.
Hum Exp Toxicol.2017, 36(11):1169-1176
Journal of Functional Foods2022, 91:105019.
Anticancer Res.2018, 38(4):2127-2135
Related and Featured Products
Planta Med. 2002 Dec;68(12):1092-6.
Kavalactones and dihydrokavain modulate GABAergic activity in a rat gastric-brainstem preparation.[Pubmed:
12494336]
METHODS AND RESULTS:
Using an in vitro neonatal rat gastric-brainstem preparation, the activity of majority neurons recorded in the nucleus tractus solitarius (NTS) of the brainstem were significantly inhibited by GABA A receptor agonist, muscimol (30 microM), and this inhibition was reversed by selective GABA A receptor antagonist, bicuculline (10 microM). Application of kavalactones (300 microg/ml) and Dihydrokavain (300 microM) into the brainstem compartment of the preparation also significantly reduced the discharge rate of these NTS neurons (39 % and 32 %, respectively, compared to the control level), and this reduction was partially reversed by bicuculline (10 microM). Kavalactones or Dihydrokavain induced inhibitory effects were not reduced after co-application of saclofen (10 microM; a selective GABA B receptor antagonist) or naloxone (100 nM; an opioid receptor antagonist). Pretreatment with kavalactones (300 microg/ml) or Dihydrokavain (300 microM) significantly decreased the NTS inhibitory effects induced by muscimol (30 microM), approximately from 51 % to 36 %.
CONCLUSIONS:
Our results demonstrated modulation of brainstem GABAergic mechanism by kavalactones and Dihydrokavain, and suggested that these compounds may play an important role in regulation of GABAergic neurotransmission.
Planta Med. 1998 Jun;64(5):458-9.
Kavain, dihydrokavain, and dihydromethysticin non-competitively inhibit the specific binding of [3H]-batrachotoxinin-A 20-alpha-benzoate to receptor site 2 of voltage-gated Na+ channels.[Pubmed:
9690349]
METHODS AND RESULTS:
The mode of action of the kava pyrones, kavain, Dihydrokavain and dihydromethysticin on the specific binding of [3H]-batrachotoxinin-A 20-alpha-benzoate to epitope 2 of voltage-dependent Na+ channels was investigated by performing saturation experiments in the presence and absence of these kava pyrones. The tested compounds significantly decreased the apparent total number of binding sites (Bmax) for [3H]-batrachotoxinin-A 20-alpha-benzoate (control: 0.5 pmol/mg protein, kava pyrones: 0.2-0.27 pmol/mg protein) with little change in the equilibrium constants (KD) for [3H]-batrachotoxin-A 20-alpha-benzoate (control: 28.2 nM, kava pyrones: 24-31 nM).
CONCLUSIONS:
The results indicate for the kava pyrones a non-competitive inhibition of the specific [3H]-batrachotoxinin-A 20-alpha-benzoate binding to receptor site 2 of voltage-gated Na+ channels.