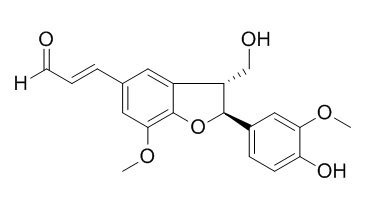
(+)-Balanophonin
(+)-Balanophonin exhibits marginal inhibition on the proliferation of OPM2 and RPMI-8226 cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Cell Biochem.2024, 125(4):e30537.
J Food Sci Technol.2022, 59(1):212-219.
Molecules2022, 27(14):4601
Journal of Functional Foods2022, 96: 105216.
Hong Kong Baptist University2023, 048330T.
Int Immunopharmacol.2020, 90:107268.
Arabian Journal of Chemistry2024, 17(3):105648
Biochem Biophys Res Commun.2017, 494(3-4):587-593
Iranian J. Pharm. Res.2021, 20(4):59-70
Virus Res.2023, 335:199199.
Related and Featured Products
Chin J Nat Med. 2013 Jul;11(4):411-4.
Isolation of cytotoxic compounds from the seeds of Crataegus pinnatifida.[Pubmed:
23845552]
To study the chemical constituents and bioactivity of the seeds of Crataegus pinnatifida.
METHODS AND RESULTS:
The chemical constituents were isolated and purified by macroporous adsorptive resin D101, silica gel, and ODS column chromatography, and preparative HPLC. Their structures were elucidated on the basis of spectroscopic methods. In addition, the cytotoxic activities of compounds 1-4 were investigated on OPM2 and RPMI-8226 cells. Four compounds were obtained and their structures were identified as (7S, 8S)-4-[2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-1-(hydroxymethyl)ethoxy]-3, 5-dimethoxybenzaldehyde (1), (+)-Balanophonin (2), erythro-guaiacylglycerol-β-coniferyl aldehyde ether (3), buddlenol A (4).
CONCLUSIONS:
Compound 1 is a novel norlignan, while compounds 1-4 exhibited marginal inhibition on the proliferation of OPM2 and RPMI-8226 cells.
Journal of Wood Science, 2008, 54(6):483-489.
Chemical constituents from Gmelina arborea bark and their antioxidant activity.[Reference:
WebLink]
Gmelina arborea Roxb. is a fast-growing species and is known to have been used in traditional Indian medicine. Chemical constituents from the bark have not been reported, although some chemical constituents from part of this plant (heartwood, leaf, and root) are known.
METHODS AND RESULTS:
In this study, the bark meal was successively extracted with acetone and methanol. Fractionation of the acetone extract with n-hexane, diethyl ether, and ethyl acetate and subsequent chromatographic separation of the fractions led to the isolation of four compounds. The diethyl ether-soluble fraction yielded tyrosol [2-(4-hydroxyphenyl)ethanol] (1); (+)-Balanophonin (2), an 8-5' neolignan, with opposite optical rotation to known (-)-balanophonin; and gmelinol (3), a known lignan. The ethyl acetate-soluble fraction afforded a new phenylethanoid glycoside to the best of our knowledge, which was identified as (-)-p-hydroxyphenylethyl[5'"-O-(3,4-dimethoxycinnamoyl)-β-d-apiofuranosyl(1'" [rightward arrow] 6')]-β-d-glucopyranoside (4). From the methanol extract, two known compounds, 2,6-dimethoxy-p-benzoquinone (5) and 3,4,5-trimethoxyphenol (6), were isolated and identified.
CONCLUSIONS:
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging assay of the identifi ed compounds indicated that 3,4,5-trimethoxyphenol (6) exhibited moderate activity.