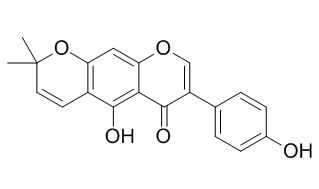
Alpinumisoflavone
Alpinumisoflavone has atheroprotective effects, may result from their ability to upregulate mechanisms promoting HDL-cholesterol and bile acid formation, it is endowed with estrogenic properties accounting, at least in part, for E. lysistemon effects. Alpinumisoflavone induces cell death, may be via repressing both the ERK/MAPK and NF-κB pathways. Alpinumisoflavone is active against both Gram-negative and Gram-positive bacteria, the minimum inhibitory concentration values obtained (MIC) ranged from 3.9 ug/mL to 125 ug/mL.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Cosmetics2021, 8(3),91.
Pharmaceuticals (Basel).2024, 17(4):462.
Int J Cosmet Sci.2019, 41(1):12-20
Food Chemistry: X2023, 101032.
Rep.Grant.Res.,Asahi Glass Foun.2023, No.119.
Phytomedicine.2022, 100:154058.
J.Korean Society of Grassland&Forage Science2023, 43(3):138-147.
Drug Dev Res.2020, doi: 10.1002
Food Chem.2024, 436:137768.
Appl. Sci.2020, 10(20),7374.
Related and Featured Products
Phytother Res. 2011 Jan;25(1):46-8.
Antibacterial activity of flavonoids from the stem bark of Erythrina caffra thunb.[Pubmed:
20623615 ]
The antibacterial activity of the stem bark of Erythrina caffra Thunb. was investigated against different bacterial strains.
METHODS AND RESULTS:
The antibacterial activity was determined by a micro broth dilution assay. Antibacterial compounds were isolated and identified using a Bruker Avance III LPO NMR spectrometer. Four known flavonoids, abyssione-V 4'-O-methyl ether, 6,8-diprenylgenistein, Alpinumisoflavone and burttinone, were isolated.
CONCLUSIONS:
All the compounds were active against both Gram-negative and Gram-positive bacteria. The minimum inhibitory concentration values obtained (MIC) ranged from 3.9 μg/mL to 125 μg/mL.
This is the first report of antibacterial activity of burttinone and the isolation of these compounds from E. caffra.
Phytother Res. 2012 Jul;26(7):1029-36.
Effects of alpinumisoflavone and abyssinone V-4'-methyl ether derived from Erythrina lysistemon (Fabaceae) on the genital tract of ovariectomized female Wistar rat.[Pubmed:
22183714]
Erythrina lysistemon is an African medicinal plant used as a palliative for problems in women. The crude extract of this plant was shown to exhibit estrogenic effects on the female rat reproductive tract and on cell cuture.
METHODS AND RESULTS:
Using classic chromatographic methods, two compounds have been isolated as major constituents of this extract: Alpinumisoflavone (1) and abyssinone V-4'-methyl ether (2). To determine whether both compounds are actives principles accounting for E. lysistemon effects, we applied the classic 3-day uterotrophic assay. We also carried out a ligand binding assay to determine whether the observed effects are estrogen receptor (ER) mediated. This study showed that whereas compound 1 displayed a fourfold preference for ERα, compound 2 bound ERα and ERβ with a same affinity. The in vivo study showed that compound 1 increased the uterine wet weight by 182.23% (p < 0.01) and 71.79% (p < 0.05) at doses of 0.1 and 1 mg/kg BW/day respectively. The uterine epithelium thickened dose-dependently. Vaginal epithelial height also increased by 369.97 and 226.76% respectively (p < 0.01). Compound 2 acted only on the vagina and increased vaginal epithelial height by 244.56% (p < 0.01) at 1 mg/kg BW/day.
CONCLUSIONS:
These results suggest that compounds 1 and 2 are endowed with estrogenic properties accounting, at least in part, for E. lysistemon effects.
J Pharm Pharmacol. 2015 Jul;67(7):990-6.
Alpinumisoflavone and abyssinone V 4'-methylether derived from Erythrina lysistemon (Fabaceae) promote HDL-cholesterol synthesis and prevent cholesterol gallstone formation in ovariectomized rats.[Pubmed:
25683903]
Erythrina lysistemon was found to improve lipid profile in ovariectomized rats. Alpinumisoflavone (AIF) and abyssinone V 4'-methylether (AME) derived from this plant induced analogous effects on lipid profile and decreased atherogenic risks. To highlight the molecular mechanism of action of these natural products, we evaluated their effects on the expression of some estrogen-sensitive genes associated with cholesterol synthesis (Esr1 and Apoa1) and cholesterol clearance (Ldlr, Scarb1 and Cyp7a1).
METHODS AND RESULTS:
Ovariectomized rats were subcutaneously treated for three consecutive days with either compound at the daily dose of 0.1, 1 and 10 mg/kg body weight (BW). Animals were sacrificed thereafter and their liver was collected. The mRNA of genes of interest was analysed by quantitative real-time polymerase chain reaction.
Both compounds downregulated the mRNA expression of Esr1, a gene associated with cholesterogenesis and cholesterol gallstone formation. AME leaned the Apoa1/Scarb1 balance in favour of Apoa1, an effect promoting high-density lipoprotein (HDL)-cholesterol formation. It also upregulated the mRNA expression of Ldlr at 1 mg/kg/BW per day (25%) and 10 mg/kg/BW per day (133.17%), an effect favouring the clearance of low-density lipoprotein (LDL)-cholesterol. Both compounds may also promote the conversion of cholesterol into bile acids as they upregulated Cyp7a1 mRNA expression.
CONCLUSIONS:
AIF and AME atheroprotective effects may result from their ability to upregulate mechanisms promoting HDL-cholesterol and bile acid formation.
Biol Pharm Bull. 2011;34(2):203-8.
Alpinumisoflavone induces apoptosis and suppresses extracellular signal-regulated kinases/mitogen activated protein kinase and nuclear factor-κB pathways in lung tumor cells.[Pubmed:
21415528 ]
Erythrina lysistemon is an African medicinal plant used as a palliative for problems in women. The crude extract of this plant was shown to exhibit estrogenic effects on the female rat reproductive tract and on cell cuture.
METHODS AND RESULTS:
Using classic chromatographic methods, two compounds have been isolated as major constituents of this extract: Alpinumisoflavone (1) and abyssinone V-4'-methyl ether (2). To determine whether both compounds are actives principles accounting for E. lysistemon effects, we applied the classic 3-day uterotrophic assay. We also carried out a ligand binding assay to determine whether the observed effects are estrogen receptor (ER) mediated. This study showed that whereas compound 1 displayed a fourfold preference for ERα, compound 2 bound ERα and ERβ with a same affinity. The in vivo study showed that compound 1 increased the uterine wet weight by 182.23% (p < 0.01) and 71.79% (p < 0.05) at doses of 0.1 and 1 mg/kg BW/day respectively. The uterine epithelium thickened dose-dependently. Vaginal epithelial height also increased by 369.97 and 226.76% respectively (p < 0.01). Compound 2 acted only on the vagina and increased vaginal epithelial height by 244.56% (p < 0.01) at 1 mg/kg BW/day.
CONCLUSIONS:
These results suggest that compounds 1 and 2 are endowed with estrogenic properties accounting, at least in part, for E. lysistemon effects.
Acta Crystallogr Sect E Struct Rep Online. 2008 Mar 14;64(Pt 4):o713.
METHODS AND RESULTS:
Alpinumisoflavone, C(20)H(16)O(5), {systematic name: 5-hydr-oxy-7-(4-hydroxy-phen-yl)-2,2-dimethyl-2H,6H-benzo[1,2-b:5,4-b']dipyran-6-one}, was obtained by demethyl-ation of the biologically active related compound, 4-O-methyl-alpinum-iso-flavone. The mol-ecular structure of Alpinumisoflavone is characterized by a fused tricyclic system that contains an approximately planar benzopyrone ring fragment. The six membered pyran ring adopts a half-chair conformation. Both ring systems show an out-of-plane twist. The dihedral angle between the mean plane of the benzopyrone system and the benzene ring is 54.29 (3)°. The mol-ecules are linked by O-H⋯O hydrogen bonds, forming a mol-ecular tape running along the b axis.
Specioside B
Catalog No: CFN95165
CAS No: 126589-95-5
Price: $368/5mg
Isolappaol C
Catalog No: CFN95225
CAS No: 929905-15-7
Price: $333/10mg
Stevia impurity (13-[(2-O-6-deoxy-beta-D-glucopyranosyl-3-O-beta-D-glucopyranosyl-beta-D-glucopyranosyl)oxy]ent-kaur-16-en-19-oic acid beta-D-glucopyranosyl ester)
Catalog No: CFN95229
CAS No: 1309929-72-3
Price: $463/5mg
15-Deoxypulic acid
Catalog No: CFN95262
CAS No: 95523-05-0
Price: $368/5mg
Cannabisin B
Catalog No: CFN95268
CAS No: 144506-17-2
Price: $413/5mg
Apigenin 6,8-di-C-alpha-L-arabinopyranoside
Catalog No: CFN95360
CAS No: 73140-47-3
Price: $318/10mg
Platycodin J
Catalog No: CFN95395
CAS No: 1325614-80-9
Price: $318/5mg
4'-Hydroxy-3',5,5',6,7,8-hexamethoxyflavone
Catalog No: CFN95407
CAS No: 85644-03-7
Price: $318/5mg
12beta-Acetoxy-3,7,11,15,23-pentaoxo-lanost-8,20-dien-26-oic acid
Catalog No: CFN95505
CAS No: 1309931-91-6
Price: $318/5mg
Ganoderic acid GS-2
Catalog No: CFN95549
CAS No: 1206781-65-8
Price: $413/5mg