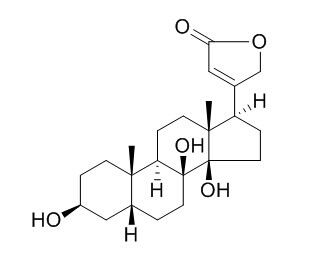
8-Hydroxydigitoxigenin
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Sci Rep.2023, 13(1):21690.
Food and Bioprocess Technology2017, 10(6):1074-1092
Phytother Res.2022, 10.1002:ptr.7592.
J Cell Physiol.2021, 236(3):1950-1966.
Chin J Pharm Anal.2019, 39(7):1217-1228
Immunopharmacol Immunotoxicol.2024, 46(4):496-508.
Biochem Pharmacol. 2020, 177:114014.
J Cell Mol Med.2023, 27(11):1592-1602.
Front Mol Neurosci.2023, 15:1083189.
Research Square2021, March 3rd.
Related and Featured Products
Planta Medica, 2007, 73(9).
Biotransformation of digitoxigenin – a plant secondary metabolite – by the fungus Cochliobolus lunatus.[Reference:
WebLink]
Cardiac glycosides are plant secondary metabolites used to treat congestive heart failure. They also exhibit a wide spectrum of biological activities, including anti-carcinogenic, acaricidal, antifilarial, molluscicidal and antibacterial properties. Biotransformation of cardenolides has been investigated either as a strategy to obtain new derivatives or to convert the A-type cardenolides into the corresponding C-type compounds, which have clinical relevance [1].
METHODS AND RESULTS:
The fungus Cochliobolus lunatus and its conidial anamorphous form Curvularia lanata are known for their capacity of hydroxylating Δ4–5 steroids at position 11β, 14α and 7α [2]. The biotransformation of digitoxigenin 1, the aglycone of digitoxin, by C. lunatus was investigated. The biotransformation reaction was carried out in a 4-day process, resulting in the isolation of four products. Their structures were elucidated by 1D and 2D NMR data as 1β-hydroxydigitoxigenin 2, 7β-hydroxydigitoxigenin 3, 8β-hydroxydigitoxigenin(8-Hydroxydigitoxigenin) 4 and digitoxigenone 5 (Fig.1). The observed hydroxylation sites are distinct from those previously described for Δ4–5 steroids in reactions with C. lunatus. The production of 4 by a biotransformation reaction is clear evidence that C. lunatus hydroxylases involved in the reaction are not affected by the steric hindrance of the 14β-OH group [3].
CONCLUSIONS:
Therefore, it is feasible to obtain a product with two vicinal hydroxyls at 8β and 14β-positions. Hydroxylation of 1 at 1β-position is of special interest, since some glycosides of 1β-hydroxydigitoxigenin have been reported to exhibit potent in vitro activity against ovarian adenocarcinoma and lung carcinoma [4].