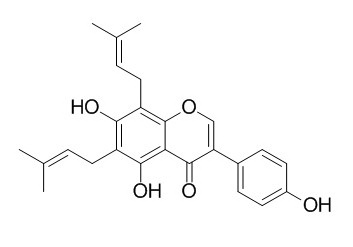
6,8-Diprenylgenistein
6,8-Diprenylgenistein has antimicrobial activity, it has anti-bacteria activity against Gram-negative and Gram-positive bacteria.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Asian Journal of Chemistry2018, 30(12):2699-2703
Asian Journal of Chemistry2014, 26(22):7811-7816
BMC Plant Biol.2023, 23(1):239.
Foods2023, 12(23), 4342.
Yakugaku Zasshi.2018, 138(4):571-579
J Nat Sc Biol Med2019, 10(2):149-156
Food Chem.2023, 427:136647.
Acta Chromatographica2016, 29(3)
Nutrients.2019, 12(1)
Biomolecules.2024, 14(4):451.
Related and Featured Products
Phytother Res. 2011 Jan;25(1):46-8.
Antibacterial activity of flavonoids from the stem bark of Erythrina caffra thunb.[Pubmed:
20623615]
The antibacterial activity of the stem bark of Erythrina caffra Thunb. was investigated against different bacterial strains.
METHODS AND RESULTS:
The antibacterial activity was determined by a micro broth dilution assay. Antibacterial compounds were isolated and identified using a Bruker Avance III LPO NMR spectrometer. Four known flavonoids, abyssione-V 4'-O-methyl ether, 6,8-Diprenylgenistein, alpinumisoflavone and burttinone, were isolated.
CONCLUSIONS:
All the compounds were active against both Gram-negative and Gram-positive bacteria. The minimum inhibitory concentration values obtained (MIC) ranged from 3.9 μg/mL to 125 μg/mL.
This is the first report of antibacterial activity of burttinone and the isolation of these compounds from E. caffra.
Caries Res. 2015;49(1):78-89.
In vitro antimicrobial activities of 1-methoxyficifolinol, licorisoflavan A, and 6,8-diprenylgenistein against Streptococcus mutans.[Pubmed:
25531232]
METHODS AND RESULTS:
Three antimicrobial flavonoids, 1-methoxyficifolinol, licorisoflavan A, and 6,8-Diprenylgenistein, were isolated from the CLE. We found that the three flavonoids and CHX had bactericidal effects on S. mutans UA159 at the concentration of ≥4 and ≥1 µg/ml, respectively. The purified compounds completely inhibited biofilm development of S. mutans UA159 at concentrations over 4 μg/ml, which was equivalent to 2 μg/ml of CHX.
CONCLUSIONS:
Confocal analysis showed that biofilms were sparsely scattered in the presence of over 4 μg/ml of the purified compounds. However, the three compounds purified from CLE showed less cytotoxic effects on NHGF cells than CHX at these biofilm-inhibitory concentrations.
J Nat Prod. 2014 Mar 28;77(3):521-6.
Pyrano-isoflavans from Glycyrrhiza uralensis with antibacterial activity against Streptococcus mutans and Porphyromonas gingivalis.[Pubmed:
24479468]
METHODS AND RESULTS:
Continuing investigation of fractions from a supercritical fluid extract of Chinese licorice (Glycyrrhiza uralensis) roots has led to the isolation of 12 phenolic compounds, of which seven were described previously from this extract. In addition to these seven metabolites, four known components, 1-methoxyerythrabyssin II (4), 6,8-Diprenylgenistein, gancaonin G (5), and isoglycyrol (6), and one new isoflavan, licorisoflavan C (7), were characterized from this material for the first time. Treatment of licoricidin (1) with palladium chloride afforded larger amounts of 7 and also yielded two new isoflavans, licorisoflavan D (8), which was subsequently detected in the licorice extract, and licorisoflavan E (9). Compounds 1-9 were evaluated for their antibacterial activities against the cariogenic Streptococcus mutans and the periodontopathogenic Porphyromonas gingivalis.
CONCLUSIONS:
Licoricidin (1), licorisoflavan A (2), and 7-9 showed antibacterial activity against P. gingivalis (MICs of 1.56-12.5 μg/mL). The most potent activity against S. mutans was obtained with 7 (MIC of 6.25 μg/mL), followed by 1 and 9 (MIC of 12.5 μg/mL). This study provides further evidence for the therapeutic potential of licorice extracts for the treatment and prevention of oral infections.