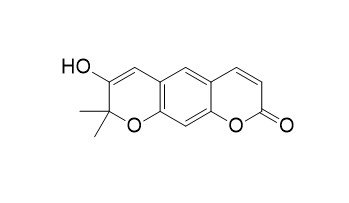
3'-Hydroxyxanthyletin
3'-Hydroxyxanthyletin showed antimycobacterial activities against Mycobacterium tuberculosis H(37)R(V) in vitro with MIC values of 16 microg/ml.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Biol Chem.2014, 289(3):1723-31
Biochem Biophys Res Commun.2020, 522(1):40-46
FEMS Microbiol Lett.2017, 364(11)
Vietnam Journal of Food Control.2022, 5(3):pp.488-497.
Molecules.2021, 26(8):2161.
Front Aging Neurosci.2018, 10:269
Foods.2020, 9(10):1348.
BMC Complement Med Ther.2023, 23(1):264.
Pamukkale Medical Journal2022, 15(4):796-803.
Drug Chem Toxicol.2020, 1-12.
Related and Featured Products
Chem Biodivers . 2010 Jul;7(7):1814-21
Secondary metabolites and antimycobacterial activities from the roots of Ficus nervosa[Pubmed:
20658670]
Bioassay-guided fractionation of the active AcOEt-soluble layer led to the isolation of two new pyranocoumarins, 3-hydroxyxanthyletin (1) and 3-methoxyxanthyletin (2), along with 22 known compounds including four simple coumarins, i.e., xanthyletin (3), umbelliferone (4), scopoletin (5), and (+)-(S)-marmesin (6); nine flavonoids, i.e., carpachromene (7), parvisoflavone B (8), alpinumisoflavone (9) genistein (10), 2'-hydroxygenistein (11), prunetin (12), cajanin (13), apigenin (14), and (2S)-naringenin (15); three benzenoids, i.e., 4-hydroxybenzaldehyde (16), vanillin (17), and (S)-lasiodiplodin (18); five steroids, i.e., ergosterol peroxide (19), a mixture of 6beta-hydroxystigmast-4-en-3-one (20) and 6beta-hydroxystigmasta-4,22-dien-3-one (21), and a mixture of beta-sitosterol (22) and stigmasterol (23); and one triterpenoid, i.e., oleanolic acid (24) from the roots of Ficus nervosa. Their structures were elucidated on the basis of extensive 1D- and 2D-NMR as well as MS analyses. Among these isolates, 3-hydroxyxanthyletin (1), genistein (10), prunetin (12), and (2S)-naringenin (15) showed antimycobacterial activities against Mycobacterium tuberculosis H(37)R(V) in vitro with MIC values of 16, 35, 30, and < or =2.8 microg/ml, respectively.
Phytochemistry. Volume 39, Issue 4,July 1995,801-804.
An alkaloid, coumarins and a triterpene from Boronia algida[Reference:
WebLink]
Examination of the aerial parts of Boronia algida has led to the isolation of six coumarins, an alkaloid and a triterpene. The coumarins were identified as ( + )-marmesin, 7-demethylsuberosin, 5-deoxyprotobruceol-II hydroperoxide, a mixture of the diastereomers of 5-deoxyprotobruceol-III 3′ξ-hydroperoxide and 3′-hydroxyxanthyletin; the alkaloid as 1-methyl-2-pentadecyl-4(1H)-quinolone and the dammarane triterpene as (E)-25-hydroperoxy-3β-hydroxydammar-20,23-diene. The latter compound and 3′-hydroxyxanthyletin are novel natural products. The chemotaxonomic significance of these isolates in the genus Boronia is discussed.