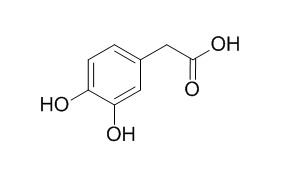
3,4-Dihydroxyphenylacetic acid
3,4-Dihydroxybenzoic acid acts as a potential ALDH inducer to prevent the alcohol-induced abnormal reaction. It has antioxidant activity, it exhibited both DPPH radical scavenging and superoxide dismutase-like activities.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Heliyon2022, 8(2):e08866.
Pak J Pharm Sci.2019, 32(6)
J Drug Target.2016, 24:1-28
The Journal of Internal Korean Medicine2015, 36(4):486-497
Mol Microbiol.2019, 112(1):317-332
Pharmaceuticals (Basel).2024, 17(8):988.
PLoS One.2021, 16(9):e0257243.
J Appl Biol Chem2023, 66:455−461
The Catharanthus Genome2022,35-83.
Molecules.2023, 28(4):1785.
Related and Featured Products
Food Res Int. 2016 Nov;89(Pt 1):716-723.
3,4-Dihydroxyphenylacetic acid is a predominant biologically-active catabolite of quercetin glycosides.[Pubmed:
28460970 ]
Since dietary flavonoid glycosides, including quercetin 4'-glucoside from onion, are poorly absorbed from the gastrointestinal tract, they are converted into smaller phenolic acids, which can be absorbed into the circulation. The purpose of this study was to compare the effects of the major phenolic acid catabolites of quercetin 4'-glucoside, including 3,4-Dihydroxyphenylacetic acid (DOPAC), 3-hydroxyphenylacetic acid, 3,4-dihydroxybenzoic acid (protocatechuic acid) and hippuric acid, on the antioxidant activity and phase II cytoprotective enzyme induction in vitro.
METHODS AND RESULTS:
Both DOPAC and protocatechuic acid, having a catechol moiety, exhibited both DPPH radical scavenging and superoxide dismutase-like activities, whereas 3-hydroxyphenyl acetic acid and hippuric acid did not. DOPAC also more potently enhanced the gene expression of several phase II drug-metabolizing enzymes than the other phenolic acid catabolites. DOPAC significantly inhibited the hydrogen peroxide-induced cytotoxicity in hepatocytes with the enhancement of the total glutathione S-transferase activity.
CONCLUSIONS:
In conclusion, DOPAC may play a key role in the antioxidative potential of the colonic lumen after the ingestion of the quercetin glycoside-rich onion.
Biosci Biotechnol Biochem. 2017 Oct;81(10):1978-1983.
3,4-Dihydroxyphenylacetic acid is a potential aldehyde dehydrogenase inducer in murine hepatoma Hepa1c1c7 cells.[Pubmed:
28828965 ]
3,4-Dihydroxyphenylacetic acid (DOPAC) is one of the major colonic microflora-produced catabolites of quercetin glycosides, such as quercetin 4'-glucoside derived from onion.
METHODS AND RESULTS:
Here, we investigated whether DOPAC modulates the aldehyde dehydrogenase (ALDH) activity and protects the cells from the acetaldehyde-induced cytotoxicity in vitro. DOPAC was shown to enhance not only the total ALDH activity, but also the gene expression of ALDH1A1, ALDH2 and ALDH3A1 in a concentration-dependent manner. DOPAC simultaneously stimulated the nuclear translocation of NFE2-related factor 2 and aryl hydrocarbon receptor. The pretreatment of DOPAC completely protected the cells from the acetaldehyde-induced cytotoxicity.
CONCLUSIONS:
The present study suggested that DOPAC acts as a potential ALDH inducer to prevent the alcohol-induced abnormal reaction.
Acta Crystallographica Section E Structure Reports Online, 2001, 57(8).
3,4-Dihydroxyphenylacetic acid[Reference:
WebLink]
METHODS AND RESULTS:
In the title compound, C8H8O4(3,4-Dihydroxyphenylacetic acid), the acetic acid side chain adopts a roughly perpendicular orientation with respect to the phenyl ring. Hydrogen bonding between carboxyl groups results in the formation of a centrosymmetric dimer. An intramolecular hydrogen bond is formed in the catechol part of the molecule. Molecules are linked together through hydrogen bonds between hydroxyl and carboxylic acid O atoms.