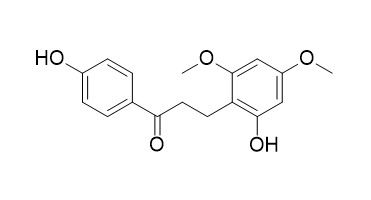
3-(2-Hydroxy-4,6-dimethoxyphenyl)-1-(4-hydroxyphenyl)-1-propanone
3-(2-Hydroxy-4,6-dimethoxyphenyl)-1-(4-hydroxyphenyl)-1-propanone(4',2-Dihydroxy-4,6-dimethoxy dihydrochalcone), an estrogen agonist, shows binding affinity for bovine uterine estrogen receptor with an IC5050 of 15 μM.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Neuroscience.2024, 559:77-90.
Drug Des Devel Ther.2023, 17:2461-2479.
Regul Toxicol Pharmacol.2024, 149:105620.
Int J Mol Sci.2019, 20(14):E3538
Phytochemistry.2021, 181:112539.
Int Immunopharmacol.2024, 141:112906.
Talanta Open2023, 7:100227
Appl. Sci. 2021, 11(10),4666.
Exp Mol Med.2020, 52(4):629-642.
Plant Cell,Tissue & Organ Culture2016, 127(1):115-121
Related and Featured Products
Planta Med. 1997 Dec;63(6):540-543.
Retrodihydrochalcones and homoisoflavones isolated from Thai medicinal plant Dracaena loureiri and their estrogen agonist activity[Pubmed:
9434606]
Biological evaluation of the extract prepared from the stem wood of Dracaena loureiri, a Thai folkloric medicine called "Chan-daeng", revealed a significant capacity to inhibit [3H]-estradiol binding to the estrogen receptor. During the course of separation, two novel (1 and 2) and two known retrodihydrochalcones (3 and 4), in addition to three known homoisoflavones (5, 6, and 7), were isolated. The structures of compounds 1 and 2 were established by NMR and MS studies by correlating their spectroscopic properties with those of 3 and 4. Each isolate was assessed for its estrogenic activity. Compounds 1 and 6 exhibited activity comparable to that of genistein and daidzein.
Planta Med . 2002 Sep;68(9):841-843.
Flavonoids and stilbenoids with COX-1 and COX-2 inhibitory activity from Dracaena loureiri[Pubmed:
12357401]
Fom the stem wood of Dracaena loureiri, a new homoisoflavanone named loureiriol (1) and eight known flavonoid and stilbenoid derivatives, including 5,7-dihydroxy-3-(4-hydroxybenzyl)-4-chromanone (2), 4,4'-dihydroxy-2,6-dimethoxydihydrochalcone (3), 2,4'-dihydroxy-4,6-dimethoxydihydrochalcone (4), 4'-hydroxy-2,4,6-trimethoxydihydrochalcone (5), 4,6,4'-trihydroxy-2-methoxydihydrochalcone (6), 4,3',5'-trihydroxystilbene (7), 4,3'-dihydroxy-5'-methoxystilbene (8) and 4-hydroxy-3',5'-dimethoxystilbene (9) were isolated. These compounds were evaluated for their inhibitory activity against the enzymes cyclooxygenase-1 and cyclooxygenase-2. Potent but non-selective activity was found for the stilbenoids 7-9 (IC(50) 1.29 - 4.92 microM) whereas weak or no activity was observed for the flavonoids 1-6.