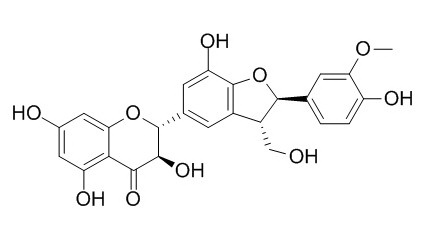
Silychristin
Silychristin is a plant growth regulator, is an anti-hepatotoxic agent, is also an inhibitor of horseradish peroxidases and lipoxygenase.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Foods.2022, 11(12):1773.
LWT2020, 126:109313
Daru.2024, 32(2):689-703.
Research Square2020, doi: 10.21203.
Evid-Based Compl Alt2020, 7202519:13
J Clin Med.2022, 11(13):3662.
J Microbiol Biotechnol.2022, 32(2):141-148.
Current Traditional Medicine, 2021, 7:326-335(10).
Biomed Pharmacother.2021, 139:111585.
J Food Sci.2021, 86(9):3810-3823.
Related and Featured Products
ongress of the Turkish Toxicology Society. 2015,10.
In vitro assessment of human CYP1A1 inhibition potential of Resveratrol and Silychristin[Reference:
WebLink]
Flavonoids are phenolic compounds with low molecule weight, which are found most plants in nature. They are so important for human health due to their biological activities such as anti-inflammatory and anti-carcinogenic effects. Cancer protective effects of flavonoids have been attributed to wide variety of mechanisms such as free radical scavenging and modifying Phase I and Phase II enzymes that activate or detoxify carcinogens. Resveratrol and Silychristin are phenolic compounds that may have roles in the reduction of cancer susceptibility. One of the possible mechanism by which resveratrol and Silychristin may exert their anti-carcinogenic effects is through an interaction by certain CYP450s.
METHODS AND RESULTS:
In this respect, the focus of this study is to determine the mechanisms of inhibition of CYP1A1, that is known to be involved in the activation of procarcinogens by resveratrol and Silychristin. Bistronic expression system that coexpress human CYP1A1 and NADPH CYP450 Reductase were used to investigate this effect. Co-expression plasmid was transformed into E. coli DH5alpha. Single colony was selected and grown in overnight culture at 30°C in LB medium. Membrane fractions were prepared and used for enzyme source. Resveratrol inhibited ethoxyresorufin O-deethylation (EROD) activity in human P450 1A1 in a dose-dependent manner with IC50 of 21 μM. Moreover, resveratrol inhibited human P450 1A1 activity in a mixed-type inhibition. In the case of Silychristin, the inhibition of human P450 1A1 by this phenolic compound was stronger than resveratrol. (IC50 15.83 μM for EROD). Similiarly, it showed mixed type inhibition.
CONCLUSIONS:
This study indicated that these phenolics were strong and selective inhibitors of CYP1A1 associated EROD activity and may be considered for use as a strong cancer chemopreventive agent in humans by the preventing malignant transformation and reducing the activations of carcinogens through inhibition of CYP1A1.
Arch Dermatol Res . 2019 Aug;311(6):477-490.
A pilot study of the UVA-photoprotective potential of dehydrosilybin, isosilybin, silychristin, and silydianin on human dermal fibroblasts[Pubmed:
31079190]
Abstract
The exposure of naked unprotected skin to solar radiation may result in numerous acute and chronic undesirable effects. Evidence suggests that silymarin, a standardized extract from Silybum marianum (L.) Gaertn. seeds, and its major component silybin suppress UVB-induced skin damage. Here, we aimed to investigate the UVA-protective effects of silymarin's less abundant flavonolignans, specifically isosilybin (ISB), Silychristin (SC), silydianin (SD), and 2,3-dehydrosilybin (DHSB). Normal human dermal fibroblasts (NHDF) pre-treated for 1 h with flavonolignans were then exposed to UVA light using a solar simulator. Their effects on reactive oxygen species (ROS), carbonylated proteins and glutathione (GSH) level, caspase-3 activity, single-strand breaks' (SSBs) formation and protein level of matrix metalloproteinase-1 (MMP-1), heme oxygenase-1 (HO-1), and heat shock protein (HSP70) were evaluated. The most pronounced preventative potential was found for DHSB, a minor component of silymarin, and SC, the second most abundant flavonolignan in silymarin. They had significant effects on most of the studied parameters. Meanwhile, a photoprotective effect of SC was mostly found at double the concentration of DHSB. ISB and SD protected against GSH depletion, the generation of ROS, carbonylated proteins and SSBs, and caspase-3 activation, but had no significant effect on MMP-1, HO-1, or HSP70. In summary, DHSB and to a lesser extent other silymarin flavonolignans are potent UVA-protective compounds. However, due to the in vitro phototoxic potential of DHSB published elsewhere, further studies are needed to exclude phototoxicity for humans as well as to confirm our results on human skin ex vivo and in vivo.
Keywords: Cell culture; Flavonolignan; Heat shock protein; Metalloproteinase-1; Oxidative damage; UVA.
Int J Mol Sci. 2015 May 26;16(6):11983-95.
Regioselective alcoholysis of silychristin acetates catalyzed by lipases.[Pubmed:
26016503]
A panel of lipases was screened for the selective acetylation and alcoholysis of Silychristin and Silychristin peracetate, respectively.
METHODS AND RESULTS:
Acetylation at primary alcoholic group (C-22) of Silychristin was accomplished by lipase PS (Pseudomonas cepacia) immobilized on diatomite using vinyl acetate as an acetyl donor, whereas selective deacetylation of 22-O-acetyl Silychristin was accomplished by Novozym 435 in methyl tert-butyl ether/ n-butanol. Both of these reactions occurred without diastereomeric discrimination of Silychristin A and B.
CONCLUSIONS:
Both of these enzymes were found to be capable to regioselective deacetylation of hexaacetyl Silychristin to afford penta-, tetra- and tri-acetyl derivatives, which could be obtained as pure synthons for further selective modifications of the parent molecule.