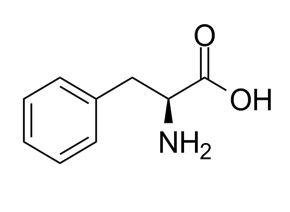
L-Phenylalanine
L-Phenylalanine has antibacterial activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Food Science and Biotechnology2023, 2023:1007
Food Research2022, 6(6): 30-38.
J Agric Food Chem.2024, acs.jafc.4c01387.
J Korean Med Ophthalmol Otolaryngol Dermatol2023, 36(1):21-39.
Chem Pharm Bull (Tokyo).2019, 67(11):1242-1247
Biomolecules.2024, 14(5):589.
Food Chem Toxicol.2020, 135:110863
Mol Immunol. 2016, 78:121-132
Kaohsiung J Med Sci.2023, 10.1002/kjm2.12764
J Chromatogr B Analyt Technol Biomed Life Sci.2019, 1113:1-13
Related and Featured Products
Biomed Khim. 2014 Jul-Aug;60(4):448-61.
Microbial synthesis of deuterium labelled L-phenylalanine with different levels of isotopic enrichment by facultative methylotrophic bacterium Brevibacterium methylicum with RMP assimilation of carbon.[Pubmed:
25249528]
METHODS AND RESULTS:
The preparative microbial synthesis of amino acids labelled with stable isotopes, including deuterium ( 2 H), suitable for biomedical applications by methylotrophic bacteria was studied using L-Phenylalanine as example. This amino acid is secreted by Gram-negative aerobic facultative methylotrophic bacteria Brevibacterium methylicum, assimilating methanol via ribulose-5-monophosphate (RMP) cycle of assimilation of carbon, The data on adaptation of L-Phenylalanine secreted by methylotrophic bacterium В. methylicum to the maximal concentration of deuterium in the growth medium with 98% 2 Н 2 O and 2% [ 2 Н]methanol, and biosynthesis of deuterium labelled L-Phenylalanine With different levels of enrichment are presented. The strain was adapted by means of plating initial cells on firm (2% agarose) minimal growth media with an increasing gradient of 2 Н 2 O concentration from 0; 24.5; 49.0; 73.5 up to 98% 2 Н 2 O followed by subsequent selection of separate colonies stable to the action of 2 Н 2 O. These colonies were capable to produce L-Phenylalanine. L-Phenylalanine was extracted from growth medium by extraction with isopropanol with the subsequent crystallization in ethanol (output 0.65 g/l).
CONCLUSIONS:
The developed method of microbial synthesis allows to obtain deuterium labelled L-Phenylalanine with different levels of isotopic enrichment, depending on concentration of 2 Н 2 O in growth media, from 17% (on growth medium with 24,5% 2 Н 2 O) up to 75% (on growth medium with 98% 2 Н 2 O) of deuterium in the molecule that is confirmed with the data of the electron impact (EI) mass- spectrometry analysis of methyl ethers of N-dimethylamino(naphthalene)-5-sulfochloride (dansyl) phenylalanine in these experimental conditions.
Biotechnol Appl Biochem . 2018 May;65(3):476-483.
Enhancement of l-phenylalanine production in Escherichia coli by heterologous expression of Vitreoscilla hemoglobin[Pubmed:
28872702]
Abstract
L-Phenylalanine is an important amino acid that is widely used in the production of food flavors and pharmaceuticals. Generally, L-Phenylalanine production by engineered Escherichia coli requires a high rate of oxygen supply. However, the coexpression of Vitreoscilla hemoglobin gene (vgb), driven bya tac promoter, with the genes encoding 3-deoxy-d-arabinoheptulosonate-7-phosphate synthetase (aroF) and feedback-resistant chorismate mutase/prephenate dehydratase (pheAfbr ), led to increased productivity and decreased demand for aeration by E. coli CICC10245. Shake-flask studies showed that vgb-expressing strains displayed higher rates of oxygen uptake, and L-Phenylalanine production under standard aeration conditions was increased. In the aerobic fermentation process, cell growth, L-Phenylalanine production, and glucose consumption by the recombinant E. coli strain PAPV, which harbored aroF, pheAfbr , and tac-vgb genes, were increased compared to that in the strain harboring only aroF and pheAfbr (E. coli strain PAP), especially under oxygen-limited conditions. The vgb-expressing strain PAPV produced 21.9% more biomass and 16.6% more L-Phenylalanine, while consuming only approximately 5% more glucose after 48 H of fermentation. This study demonstrates a method to enhance the L-Phenylalanine production by E. coli using less intensive and thus more economical aeration conditions.
Keywords: Escherichia coli; Vitreoscilla hemoglobin; dissolved oxygen; feedback resistant; L-Phenylalanine; vgb.
Biotechnol Bioeng. 2014 Jul;111(7):1406-16.
Improvement of constraint-based flux estimation during L-phenylalanine production with Escherichia coli using targeted knock-out mutants.[Pubmed:
24449451]
METHODS AND RESULTS:
Fed-batch production of the aromatic amino acid L-Phenylalanine was studied with recombinant Escherichia coli strains on a 15 L-scale using glycerol as carbon source. Flux Variability Analysis (FVA) was applied for intracellular flux estimation to obtain an insight into intracellular flux distribution during L-Phenylalanine production. Variability analysis revealed great flux uncertainties in the central carbon metabolism, especially concerning malate consumption. Due to these results two recombinant strains were genetically engineered differing in the ability of malate degradation and anaplerotic reactions (E. coli FUS4.11 ΔmaeA pF81kan and E. coli FUS4.11 ΔmaeA ΔmaeB pF81kan). Applying these malic enzyme knock-out mutants in the standardized L-Phenylalanine production process resulted in almost identical process performances (e.g., L-Phenylalanine concentration, production rate and byproduct formation). This clearly highlighted great redundancies in central metabolism in E. coli.
CONCLUSIONS:
Uncertainties of intracellular flux estimations by constraint-based analyses during fed-batch production of L-Phenylalanine were drastically reduced by application of the malic enzyme knock-out mutants.
Microbiol Res. 2014 Sep-Oct;169(9-10):675-85.
A study of the antibacterial activity of L-phenylalanine and L-tyrosine esters in relation to their CMCs and their interactions with 1,2-dipalmitoyl-sn-glycero-3-phosphocholine, DPPC as model membrane.[Pubmed:
24667307]
Cationic amino acid-based surfactants are known to interact with the lipid bilayer of cell membranes resulting in depolarization, lysis and cell death through a disruption of the membrane topology.
METHODS AND RESULTS:
A range of cationic surfactant analogues derived from L-Phenylalanine (C1-C20) and L-Tyrosine (C8-C14) esters have been synthesized and screened for their antibacterial activity.