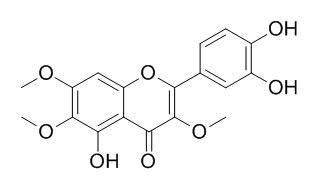
Chrysosplenol D
Chrysosplenol D, an efflux pump inhibitor that can potentiate the activity of commercially important antibiotics and antimalarials. Chrysosplenol D has anti-inflammatory, antimalarial, antibacterial and antifungal activities; it also exerts its anti-proliferative effect on tsFT210 cells through inhibiting cell cycle and inducing apoptosis, it may as a new cell cycle inhibitor.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Front Cell Infect Microbiol.2018, 8:292
Antioxidants (Basel).2021, 10(8):1300.
J Biotechnol.2020, 318:10-19.
Int J Food Sci Nutr.2019, 70(7):825-833
Appl. Sci. 2021, 11(8),3437.
Asian Pac J Tropical Bio.2020, 10(6):239-247
Int J Mol Sci.2020, 21(19):7209.
Molecules.2024, 29(5):1171.
Phytomedicine.2020, 153440.
Mol Med Rep.2023 Oct;28(4):193.
Related and Featured Products
Plant Cell Rep. 1992 Nov;11(12):637-40.
Antimalarial activity of Artemisia annua flavonoids from whole plants and cell cultures.[Pubmed:
24213368 ]
Cell suspension cultures developed from Artemisia annua exhibited antimalarial activity against Plasmodium faldparum in vitro both in the n-hexane extract of the plant cell culture medium and in the chloroform extract of the cells.
METHODS AND RESULTS:
Trace amounts of the antimalarial sesquiterpene lactone artemisinin may account for the activity of the n-hexane fraction but only the methoxylated flavonoids artemetin, chrysoplenetin, chrysosplenol-D and cirsilineol can account for the activity of the chloroform extract. These purified flavonoids were found to have IC50 values at 2.4 - 6.5 × 10(-5)M against P. falciparum in vitro compared with an IC50 value of about 3 × 10(-8)M for purified artimisinin.
CONCLUSIONS:
At concentrations of 5 × 10(-6)M these flavonoids were not active against P. falciparum but did have a marked and selective potentiating effect on the antiplasmodial activity of artemisinin.
Phytochemistry, 1989, 28(9):2323-7.
Antimicrobial flavonoids from Psiadia trinervia and their methylated and acetylated derivatives.[Reference:
WebLink]
From a dichloromethane extract and a hydrolysed methanolic extract from the leaves of Psiadia trinervia, 13 3-methylated flavonols have been isolated.
METHODS AND RESULTS:
Their structures were established by the usual spectroscopic methods (UV, EIMS, 1H and 13C NMR). Ayanin, casticin, chrysosplenol-D and 5,7,4′-trihydroxy-3,8-dimethoxyfiavone were responsible for the antifungal activity found in the preliminary screening. Chrysosplenol D, isokaempferide, 5,7,4′- trihydroxy-3,3′-dimethoxyflavone and 5,7,4′-trihydroxy-3,8-dimethoxyflavone displayed antibacterial activity. Twenty-nine derivatives were prepared by permethylation and selective methylation of the free hydroxyl group at C-5.
CONCLUSIONS:
The antimicrobial activities of the isolates and derivatives were determined by bioautographic assays using C. cucumerinum and B. cereus as test organisms.
Nat Prod Res . 2021 Dec;35(24):5909-5913.
Anti-inflammatory effect of methoxyflavonoids from Chiliadenus montanus ( Jasonia Montana) growing in Egypt[Pubmed:
32746641]
Abstract
Chiliadenus montanus is a medicinal plant that grows in Sinai Peninsula in Egypt. Phytochemical investigation of C. montanus methanolic extract led to the isolation of five methoxy flavonoids; Chrysosplenol-D (1), 5,7,4'-trihydroxy- 3,3'-dimethoxy flavone (2), 5,7-dihydroxy -3,3',4'-trimethoxyflavone (3), Bonanzin (4), 3,5,6,7,4'-pentamethoxy flavone (5), a sesquiterpene, Cryptomeridiol (6) and stigmast-5,22-dien-3-O-β-D-glucopyranoside (7). The anti-inflammatory activity of compounds 2 and 5 was assessed in vitro on CaCo2 cells stimulated by lipopolysaccharide (LPS). Both compounds downregulated LPS-induced expression of inflammatory cytokines; tumor necrosis factor alpha (TNFα), interleukin 1β (IL1β), nuclear factor kappa B (NFκB), cyclooxygenase 1 (Cox1), cyclooxygenase 2 (Cox2), and 5-lipoxygenase (5Lox). In vivo, both compounds significantly decreased paw edema thickness in rats relative to carrageenan, showing better anti-inflammatory activity than celecoxib (36.98%) after 1 h (46.60% and 48.11%, respectively). An in silico study was performed, where both compounds were docked into the active site of the crystal structure of the human Cox2 enzyme.
Keywords: Chiliadenus montanus; colorectal cancer; cytokines; docking; methoxyflavonoids.
Int J Mol Sci . 2020 Jun 8;21(11):4090.
Chrysosplenol d, a Flavonol from Artemisia annua, Induces ERK1/2-Mediated Apoptosis in Triple Negative Human Breast Cancer Cells[Pubmed:
32521698]
Abstract
Triple negative human breast cancer (TNBC) is an aggressive cancer subtype with poor prognosis. Besides the better-known artemisinin, Artemisia annua L. contains numerous active compounds not well-studied yet. High-performance liquid chromatography coupled with diode-array and mass spectrometric detection (HPLC-DAD-MS) was used for the analysis of the most abundant compounds of an Artemisia annua extract exhibiting toxicity to MDA-MB-231 TNBC cells. Artemisinin, 6,7-dimethoxycoumarin, arteannuic acid were not toxic to any of the cancer cell lines tested. The flavonols Chrysosplenol D and casticin selectively inhibited the viability of the TNBC cell lines, MDA-MB-231, CAL-51, CAL-148, as well as MCF7, A549, MIA PaCa-2, and PC-3. PC-3 prostate cancer cells exhibiting high basal protein kinase B (AKT) and no ERK1/2 activation were relatively resistant, whereas MDA-MB-231 cells with high basal ERK1/2 and low AKT activity were more sensitive to Chrysosplenol D treatment. In vivo, Chrysosplenol D and casticin inhibited MDA-MB-231 tumor growth on chick chorioallantoic membranes. Both compounds induced mitochondrial membrane potential loss and apoptosis. Chrysosplenol D activated ERK1/2, but not other kinases tested, increased cytosolic reactive oxygen species (ROS) and induced autophagy in MDA-MB-231 cells. Lysosomal aberrations and toxicity could be antagonized by ERK1/2 inhibition. The Artemisia annua flavonols Chrysosplenol D and casticin merit exploration as potential anticancer therapeutics.
Keywords: apoptosis; artemisia annua; casticin; cell cycle; chick chorioallantoic membrane assay; Chrysosplenol D; methoxylated flavonoids; mitogen-activated protein kinase; triple negative breast cancer.
Fitoterapia . 2018 Mar;125:191-198.
Improving anti-trypanosomal activity of alkamides isolated from Achillea fragrantissima[Pubmed:
29108932]
Abstract
In previous studies the aerial parts of Achillea fragrantissima were found to have substantial antileishmanial and antitrypanosomal activity. A bioassay-guided fractionation of a dichloromethane extract yielded the isolation of the essential anti-trypanosomal compounds of the plant. Seven sesquiterpene lactones (including Achillolide-A), two flavonoids, chrysosplenol-D and chrysosplenetine, and four alkamides (including pellitorine) were identified. This is the first report for the isolation of the sesquiterpene lactones 3 and 4, chrysosplenetine and the group of alkamides from this plant. Bioevaluation against Trypanosoma brucei brucei TC221 (T.b brucei) using the Alamar-Blue assay revealed the novel alkamide 13 to have an IC50 value of 40.37μM. A compound library, derived from the alkamide pellitorine (10), was synthesized and bioevaluated in order to find even more active substances. The most active compounds 26 and 27 showed activities in submicromolar concentrations and selectivity indices of 20.1 and 45.6, respectively, towards macrophage cell line J774.1. Toxicity of 26 and 27 was assessed using the greater wax moth Galleria mellonella larvae as an in vivo model. No significant toxicity was observed for the concentration range of 1.25-20mM.
Keywords: Achillea fragrantissima; Alkamides; Anti-trypanosomal; Flavonoids; Sesquiterpene lactones.
Toxicol Appl Pharmacol. 2015 Apr 17.
Flavonoids casticin and chrysosplenol D from Artemisia annua L. inhibit inflammation in vitro and in vivo.[Pubmed:
25891417]
The aim of our experiments was to investigate the anti-inflammatory properties of casticin and Chrysosplenol D, two flavonoids present in Artemisia annua L.
METHODS AND RESULTS:
Topical inflammation was induced in ICR mice using croton oil. Mice were then treated with casticin or Chrysosplenol D. Cutaneous histological changes and edema were assessed. ICR mice were intragastrically administrated with casticin or Chrysosplenol D followed by intraperitoneal injection of lipopolysaccharide (LPS). Mouse Raw264.7 macrophage cells were incubated with casticin or Chrysosplenol D. Intracellular phosphorylation was detected, and migration was assessed by trans-well assay. HT-29/NFκB-luc cells were incubated with casticin or Chrysosplenol D in the presence or absence of LPS, and NF-κB activation was quantified. In mice, administration of casticin (0.5, 1 and 1.5μmol/cm2) and Chrysosplenol D (1 and 1.5μmol/cm2) inhibited croton oil-induced ear edema (casticin: 29.39-64.95%; Chrysosplenol D: 37.76-65.89%, all P<0.05) in a manner similar to indomethacin (0.5, 1 and 1.5μmol/cm2; 55.63-84.58%). Casticin (0.07, 0.13 and 0.27mmol/kg) and Chrysosplenol D (0.07, 0.14 and 0.28mmol/kg) protected against LPS-induced systemic inflammatory response syndrome (SIRS) in mice (all P<0.05), in a manner similar to dexamethasone (0.03mmol/kg). Casticin and Chrysosplenol D suppressed LPS-induced release of IL-1 beta, IL-6 and MCP-1, inhibited cell migration, and reduced LPS-induced IκB and c-JUN phosphorylation in Raw264.7 cells. JNK inhibitor SP600125 blocked the inhibitory effect of Chrysosplenol D on cytokine release.
CONCLUSIONS:
The flavonoids casticin and Chrysosplenol D from A. annua L. inhibited inflammation in vitro and in vivo.
J Nat Prod. 2008 Nov;71(11):1961-2.
Direct synthesis of chrysosplenol D.[Pubmed:
18855445]
An aldol condensation and an Algar-Flynn-Oyamada oxidative cyclization were key steps in the direct synthesis of Chrysosplenol D, an efflux pump inhibitor that can potentiate the activity of commercially important antibiotics and antimalarials.
Nat Prod Res. 2014;28(11):812-8.
A new α-glucosidase inhibitor from Achillea fragrantissima (Forssk.) Sch. Bip. growing in Egypt.[Pubmed:
24666348]
α-Glucosidase inhibitors (AGIs) represent a class of oral antidiabetic drugs that delay the absorption of ingested carbohydrates, reducing the postprandial glucose and insulin peaks to reach normoglycaemia.
METHODS AND RESULTS:
In this study, a bioassay-guided fractionation of the ethanolic extract of the aerial parts of Achillea fragrantissima (Forssk.) Sch. Bip. growing in Egypt led to the isolation of a new potent AGI; acacetin-6-C-(6″-acetyl-β-D-glucopyranoside)-8-C-α-L-arabinopyranoside (5) alongside with four known compounds: chondrillasterol (1), quercetin-3,6,7-trimethyl ether (chrysosplenol-D) (2), isovitexin-4'-methyl ether (3) and isovitexin (4). The structure of the new compound (5) was elucidated on the basis of its spectral data, including HR-FAB-MS, UV, (1)H NMR, (13)C NMR, (1)H-(1)H COSY, HSQC and HMBC. The new compound (5) exhibited the most significant α-glucosidase inhibitory activity (IC₅₀ 1.5 ± 0.09 μg/mL).
CONCLUSIONS:
Under the assay conditions, all the tested compounds were more potent than the positive control acarbose (IC50 224 ± 2.31 μg/mL).
J Asian Nat Prod Res. 2005 Aug;7(4):615-26.
Flavonoids from Vitex trifolia L. inhibit cell cycle progression at G2/M phase and induce apoptosis in mammalian cancer cells.[Pubmed:
16087636 ]
Six flavonoids, persicogenin (1), artemetin (2), luteolin (3), penduletin (4), vitexicarpin (5) and Chrysosplenol D (6), have been isolated for the first time as new cell cycle inhibitors from Vitex trifolia L., a Chinese folk medicine used to treat cancers, through a bioassay-guided separation procedure. They were identified by spectroscopic methods.
METHODS AND RESULTS:
The inhibitory effects of 1-6 on the proliferation of mammalian cancer cells have been evaluated by the SRB (sulforhodamine B) method and their effects on cell cycle and apoptosis investigated by flow cytometry with the morphological observation under light microscope and by agarose-gel electrophoresis to detect internucleosomal DNA fragmentation. Compounds 1-6 inhibited the proliferation of mouse tsFT210 cancer cells with the IC50s (microg ml(-1)) > 100 (inhibition rate at 100 microg ml(-1), 47.9%) for 1, >100 (inhibition rate at 100 microg ml(-1), 49.6 %) for 2, 10.7 for 3, 19.8 for 4, 0.3 for 5, and 3.5 for 6. Flow cytometric investigations for 1-6 demonstrated that 1-5 mainly inhibited cell cycle at the G2/M phase in a dose-dependent manner with a weak induction of apoptosis on the tsFT210 cells, while 6 induced mainly apoptosis of the same tsFT210 cells also in a dose-dependent manner together with a weak inhibition of the cell cycle at the G0/G1 and G2/M phases, demonstrating that 1-6 exert their anti-proliferative effect on tsFT210 cells through inhibiting cell cycle and inducing apoptosis. In contrast to the cell cycle G2/M phase inhibitory main effect on tsFT210 cells, 5 induced mainly apoptosis on human myeloid leukemia K562 cells with a weak inhibition of the cell cycle at the G2/M phase.
CONCLUSIONS:
The present result provides flavonoids 1-6 as new cell cycle inhibitors and 1 and 4 as new anticancer flavonoids, which not only provide the first example of cell cycle G2/M phase inhibitory and apoptosis-inducing constituents of V. trifolia L. but also explain the use of Vitex trifolia L. by Chinese people to treat cancers.