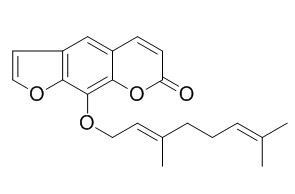
8-Geranyloxypsoralen
8-Geranyloxypsoralen inhibits β-secretase (BACE1) activity in non-competitive manner, with the IC(50) values <25.0 uM, it can induce vasorelaxation on rat arterial tissues. 8-Geranyloxypsoralen possesses nematicidal activities, the median lethal concentrations (LC(50)) is 188.3 mg/ L against B. xylophilus and is 117.5 mg/L against P. redivivus.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Phytomedicine.2020, 153440.
Plant Cell Tiss Org2020, 1-16
Srinakharinwirot University2023, 2669.
Universidade Estadual Paulista2017, 42785
Molecules.2019, 24(11):E2044
Food Funct.2022, doi: 10.1039
Fitoterapia.2022, 105141.
Res Rep Urol.2022, 14:313-326.
J Food Sci Technol.2019, 56(5):2712-2720
Fermentation2023, 9(10), 889
Related and Featured Products
Nat Prod Res. 2008 May 20;22(8):666-71.
Nematicidal coumarins from Heracleum candicans Wall.[Pubmed:
18569707 ]
The root extract of Heracleum candicans Wall. exhibited antagonistic activities against nematodes Bursaphelenchus xylophilus (Steiner et Buhrer) Nickle and Panagrellus redivivus (Linn.) Goodey.
METHODS AND RESULTS:
Through bioassay-guided fractionations, three coumarins were obtained from the extract of H. candicans and determined to be 8-Geranyloxypsoralen (1), imperatorin (2), and heraclenin (3) based on spectra data. All three compounds possessed nematicidal activities against the two tested nematodes. The median lethal concentrations (LC(50)) of compounds 1-3 at 72 h were 188.3, 161.7, and 114.7 mg L(-1) respectively against B. xylophilus and were 117.5, 179.0, and 148.7 mg L(-1) respectively against P. redivivus.
CONCLUSIONS:
This is the first report about species in the Umbelliferae family that possesses nematicidal activity.
Phytomedicine. 2014 Apr 15;21(5):586-94.
Vasodilation and radical-scavenging activity of imperatorin and selected coumarinic and flavonoid compounds from genus Casimiroa.[Pubmed:
24309287]
Hypertension is a very widespread condition which is not strictly considered as an illness but if not countered, progressively causes damage to all tissues and loss in their functionality. For this reason the find of new antihypertensive agents is prominent and medicinal plants and their derivatives are valuable for the purpose. The genus Casimiroa (Rutaceae) includes plants from Central America and Mexico; among these, Casimiroa edulis Llave et Lex. and Casimiroa pubescens Ramirez are the most relevant species, even for their medicinal uses. The decoction of leaves and seeds is traditionally taken as a tea mainly to lower blood pressure.
METHODS AND RESULTS:
The object of this research was the study of vascular activity of coumarinic and flavonoid compounds isolated from seeds of Casimiroa spp. in comparison with Casimiroa edulis and Casimiroa pubescens extracts. The phenolic compounds isolated from Casimiroa were herniarin (Her), imperatorin (Imp), 8-Geranyloxypsoralen (GOP) and 5,6,2',3',4'-pentamethoxyflavone (PMF). All these compounds induced vasorelaxation on rat arterial tissues although with different effectiveness. To study the cellular mechanisms of the vasorelaxation exhibited by imperatorin, we used selective inhibitors of different receptors and enzymes, such as atropine, pyrilamine, nifedipine, L-NAME and DETC. In a further step of this research, we evaluated the radical-scavenging activity of Casimiroa extracts and isolated compounds by means of DPPH assay. In general, we observed that the scavenging activities increased in a concentration-dependent manner for all substances. The phenolic compounds highlight a synergism of vasodilation and antioxidant activity which may be very useful in the management of cardiovascular diseases.
CONCLUSIONS:
Among the evaluated compounds, imperatorin shows a significant vasorelaxant activity even higher than acetylcholine and similar to nitrite, and also useful antiradical capabilities. All these properties suggest its possible role against hypertension and vasculopathies, even if in vivo studies are needed to determine the actual applications.
Bioorg Med Chem. 2006 Jun 1;14(11):3865-71.
Synthesis of 8-geranyloxypsoralen analogues and their evaluation as inhibitors of CYP3A4.[Pubmed:
16481174]
Furanocoumarins have been shown to inhibit CYP3A4 in vitro with varying degrees of potency.
METHODS AND RESULTS:
In this study, we report the effects of a series of novel furanocoumarins based on the naturally occurring derivative 8-geranylepoxypsoralen which has been shown to be a more potent inhibitor of CYP3A4 than its 5-position-substituted counterpart bergamottin. Compounds were designed, synthesised and tested for their ability to inhibit CYP3A4 activity in human liver microsomes using testosterone as the marker substrate. Both the saturated and unsaturated phenolic furanocoumarin derivatives were found to be inactive. However, the 8-alkyloxy-furanocoumarin analogues were shown to inhibit CYP3A4 activity in a dose dependent manner, with IC(50) values ranging from 0.78+/-0.11 to 3.93+/-0.53 microM.
CONCLUSIONS:
The reduced furan derivative dihydro-8-Geranyloxypsoralen showed a 4-fold decrease in inhibitory potency, suggesting that the furan moiety plays a role in the interaction between these compounds and CYP3A4.
Bioorg Med Chem. 2012 Jan 15;20(2):784-8.
Structure-activity relationships for naturally occurring coumarins as β-secretase inhibitor.[Pubmed:
22222157]
The present study was demonstrated to evaluate the effects of naturally occurring coumarins (NOCs) including simple coumarins, furanocoumarins, and pyranocoumarins on the inhibition of β-secretase (BACE1) activity.
METHODS AND RESULTS:
Of 41 NOCs examined, some furanocoumarins inhibited BACE1 activity, but simple coumarins and pyranocoumarins did not affect. The most potent inhibitor was 5-geranyloxy-8-methoxypsoralen (31), which has an IC(50) value of 9.9 μM. Other furanocoumarin derivatives, for example, 8-geranyloxy-5-methoxypsoralen (35), 8-Geranyloxypsoralen (24), and bergamottin (18) inhibited BACE1 activity, with the IC(50) values <25.0 μM.
CONCLUSIONS:
Analyses of the inhibition mechanism by Dixon plots and Cornish-Bowden plots showed that compounds 18, 31 and 35 were mixed-type inhibitor. The kinetics of inhibition of BACE1 by coumarins 24 was non-competitive inhibitors.